
The severity and duration of the stresses can be controlled precisely using simple protocols in software.
Abiotic Stresses
Qubit’s PlantScreen™ conveyor and XYZ high throughput plant screening systems can be used to both induce defined stresses and identify the degree to which plants are resistant or susceptible. Analysis of chlorophyll fluorescence kinetics is key to identifying the precise point at which plants begin to respond to stress conditions and the degree to which these conditions inhibit photochemical efficiency. RGB and morphometric analyses quantify effects on growth, whereas NIR and thermal imaging indicate water status and stomatal closure.
Our automated watering and weighing protocols can be used to induce various stress conditions including:
Biotic Stresses
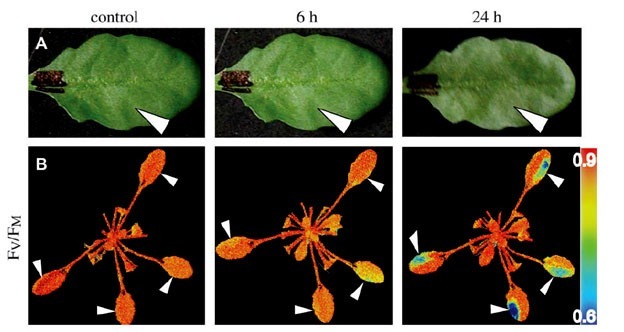
Infection of crop plants by pathogens is a major factor contributing to reduction in crop yields. The PlantScreen™ conveyor and XYZ systems allow accurate quantification of the degree to which crop varieties are susceptible to pathogen attack, providing an extremely valuable tool in breeding programs and screening of GMOs. For example, RGB analysis quantifies the time course of plant fungal infection, while measurement of chlorophyll fluorescence kinetics shows how the infection limits photochemical efficiency and potential yield. Repeated morphometric analysis quantifies reduced growth and effects on plant architecture.

Plant Growth Analysis
PlantScreen™ systems incorporate high resolution cameras to monitor numerous aspects of plant growth and morphology throughout the development of plants. Each plant, whether it is in a pot and measured separately, or in a tray and measured together with other samples, is tracked individually so that the time course of changes in growth parameters and plant architecture, can analyzed with respect to developmental stage. We offer a unique scanning camera system, that can recreate the 3D structure of plants, with greater accuracy and higher resolution than laser-scattering methods. Both monocots and dicots may be screened. PlantScreen™ systems are available in several sizes and configurations to track the growth of very small (e.g. Arabidopsis) and very large (e.g. mature corn) samples.
Crop and Field Studies
Numerous configurations of PlantScreen™ systems can be custom-designed to operate in field conditions for screening a wide variety of crops and natural vegetation. We build systems to image plants over small transects as well as over hundreds of square meters. Plant growth and physiological data are collated with environmental data (pCO2, PAR, Temperature, Relative Humidity, Wind Speed) so that the dynamic changes common in field situations can be included in multifactoral analyses of plant growth and development.
Field systems may be static or mobile, depending on the situation. We work very closely with our clients to ensure that immediate and longer terms needs are considered when designing systems, taking into account the potential evolution of projects and the additional features that may be required as applications expand.
All field systems are constructed to withstand the particular environments in which they are used, including both high and low temperatures and high and low relative humidities. We can even build submersible systems to monitor plants during simulated flooding. The PlantScreen™ components are rugged and guaranteed to function unattended once the screening protocols have been established and set in motion.
As well as large scale phenomic screening systems, Qubit offers small field portable chlorophyll fluorescence imaging systems, and hand-held non imaging devices for spot measurements of chlorophyll fluorescence and hyperspectral parameters, that can be used for individual plant analysis and verification.



Trait Discovery
Using the PlantScreen™ systems, measurements of plant growth rates and architecture may be correlated with a wide variety of non-invasive measurements such as chlorophyll fluorescence kinetics, hyperspectral indices, thermal imaging and NIR imaging to uncover the underlying physiological and metabolic bases for variations in plant performance. Differences in the growth and physiological responses of plant varieties subjected to multifactoral environmental conditions can lead to the discovery of traits beneficial to the growth of crops under extremes of water limitation, high light, high and low temperatures etc. High throughput PlantScreen™ Systems can process hundreds to thousands of plants per day all at the same stage of development, and all under stringently controlled conditions. Analytical software identifies those plants that perform either very well or very poorly, providing a primary screen for later analyses to determine the genetic bases of the responses.
Nutrient Stress and Ecotoxicology
The PlantScreen™ system has the option for delivery of specific nutrient regimes to plants so that effects of nutrient deficiency can be studied throughout the growth cycle. In addition, the nutrient delivery system can be used to provide doses of heavy metals, or other potentially toxic substances, so that tolerance or susceptibility to such stresses may be screened. The systems may be used with aquatic plants, such as duckweeds (Lemnodeae), for quantitative testing of ecotoxilogical challenges, as may be required international regulatory bodies. Relevant measurements include chlorophyll kinetic imaging to determine how nutrient or chemical challenges affect the photosynthetic physiology and efficiency of the plants. RGB and morphometric images analysis enables the monitoring of plant color and growth parameters. All data are immediately available for detailed analysis within the PlantScreen™ software, or for numerical download to other programs for mathematical and statistical analysis.


Functional Genomics
The high-throughput capacity of PlantScreen™ systems enables the capture and collation of a huge amount of data representing various aspects of the phenotypes of plants under study. Depending on the size of the PlantScreen™ system, and the various imaging protocols that may be selected, hundreds to tens of thousands of plants may be screened repeatedly throughout different stages of growth. Small dicots to large maize plants may be imaged with equal effectiveness. Various aspects of the plants’ growth rate and growth character are measured and collated, including height, compactness, eccentricity, leaf perimeter, leaf area, circle diameter etc. Together, these factors represent the plants’ distinct phenomic identities which can be used to separate a population of plants into phenomic sub-groups. Such analyses are important for gene discovery.
Root Phenotyping
Numerous difficulties are associated with the non-invasive measurement and characteristic of root growth. Several techniques are available, including growth of roots in transparent pots, or in gels, so that standard imaging techniques may be applied. Low dose x-rays may also be used to visualize roots grown in hydroponic beads. The choice of technique is determined by the degree to which the research scientist requires that the plants are grown under natural conditions. This requirement may offset by the paucity of data attainable in soil-grown roots, especially at early stages of development. At Qubit, we work closely with our clients to determine the optimum approach to all phenotyping investigations, so that the researcher is made aware of the advantages and pitfalls of various techniques. We can offer unique solutions to root-scanning problems, and include soil water status measurements to determine both the amount of water absorbed by the roots and the location of maximum uptake.


